What Other Properties Could Mendeleev Examine to Determine Families of Elements?
Periodic trends in properties such as diminutive size and ionic size, ionization free energy, electron analogousness, and electronegativity illustrate the strong connection between the chemic properties and the reactivity of the elements and their positions in the periodic table. In this section, we explore that connectedness by focusing on two periodic properties that correlate strongly with the chemical behavior of the elements: valence electron configurations and Mulliken electronegativities.
The Chief Group Elements
We have said that elements with the aforementioned valence electron configuration (i.e., elements in the same column of the periodic table) often have similar chemical science. This correlation is particularly axiomatic for the elements of groups i, two, 3, xiii, sixteen, 17, and 18. The intervening families in the p block (groups 14 and 15) straddle the diagonal line separating metals from nonmetals. The lightest members of these two families are nonmetals, so they react differently compared to the heaviest members, which are metals. We begin our survey with the brine metals (grouping 1), which contain only a single electron exterior a noble gas electron configuration, and cease with the noble gases (group xviii), which have full valence electron shells.
Grouping 1: The Alkali Metals
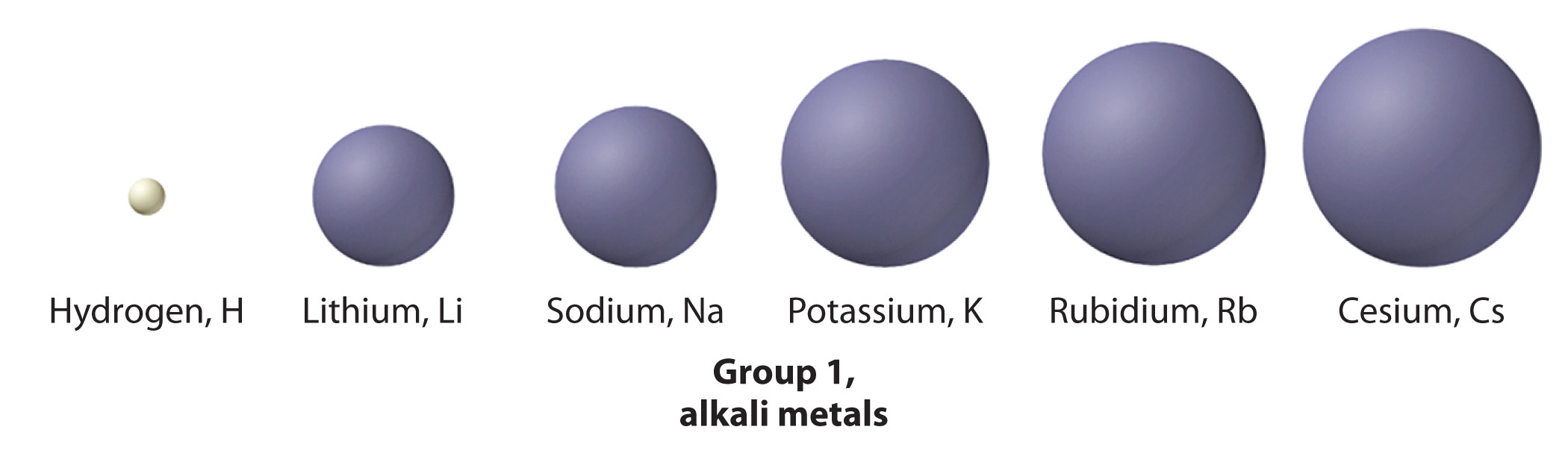
The elements of group 1 are called the alkali metals. Brine (from the Arabic al-qili, meaning "ashes of the saltwort constitute from table salt marshes") was a general term for substances derived from wood ashes, all of which possessed a bitter taste and were able to neutralize acids. Although oxides of both group ane and grouping ii elements were obtained from forest ashes, the alkali metals had lower melting points.
Potassium and sodium were first isolated in 1807 by the British chemist Sir Humphry Davy (1778–1829) by passing an electrical current through molten samples of potash (ThoutwoCOthree) and soda ash (Na2COthree). The potassium burst into flames as soon as it was produced because it reacts readily with oxygen at the higher temperature. However, the group i elements, like the group 2 elements, go less reactive with air or water equally their diminutive number decreases. The heaviest element (francium) was not discovered until 1939. It is and so radioactive that studying its chemistry is very difficult.
The alkali metals have ns one valence electron configurations and the lowest electronegativity of any group; hence they are often referred to as beingness electropositive elements. As a result, they have a strong tendency to lose their single valence electron to form compounds in the +1 oxidation state, producing the EX monohalides and the E2O oxides.
Because they are so reactive, pure group 1 elements are powerful reducing agents that are used in lithium batteries and cardiac pacemakers. Sodium salts such as common tabular array salt (NaCl), baking soda (NaHCO3), soda ash (Na2CO3), and caustic soda (NaOH) are important industrial chemicals. Other compounds of the alkali metals are important in biology. For example, because potassium is required for institute growth, its compounds are used in fertilizers, and lithium salts are used to treat manic-depressive, or bipolar, disorders.
Potassium burning. A piece of potassium dropped in a chalice of water will burn as it skips across the top of the h2o.
Group 2: The Alkali metal Earth Metals
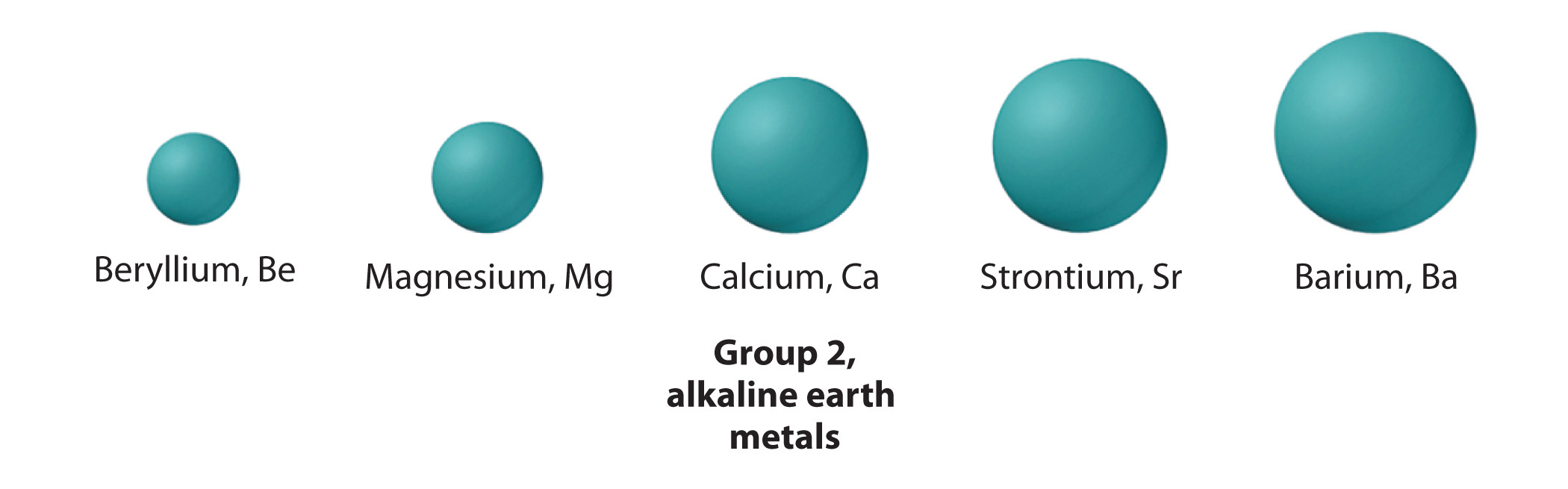
The elements of group 2 are collectively referred to as the alkaline globe metals, a name that originated in the Middle Ages, when an "globe" was defined equally a substance that did non melt and was non transformed by fire. Alkalis that did not melt hands were called "alkali metal earths."
Retrieve that the trend in about groups is for the lightest member to have backdrop that are quite different from those of the heavier members. Consistent with this trend, the properties of the lightest element—in this example, glucinium—tend to be dissimilar from those of its heavier congeners, the other members of the group. Beryllium is relatively unreactive only forms many covalent compounds, whereas the other grouping members are much more reactive metals and course ionic compounds. As is the example with the alkali metals, the heaviest element, radium, is highly radioactive, making its size hard to mensurate. Radium was discovered in 1902 by Marie Curie (1867–1934; Nobel Prize in Chemistry 1903 and Nobel Prize in Chemistry 1911), who, with her husband, Pierre, isolated 120 mg of radium chloride from tons of residues from uranium mining. (For more information well-nigh radioactivity, see Chapter i "Introduction to Chemistry", Section 1.five "The Cantlet".)
All the alkaline earth metals have ns 2 valence electron configurations, and all accept electronegativities less than 1.6. This ways that they behave chemically as metals (although beryllium compounds are covalent) and lose the two valence electrons to class compounds in the +2 oxidation country. Examples include the dihalides (EX2) and the oxides (EO).
Compounds of the group 2 elements take been commercially important since Egyptian and Roman times, when blocks of limestone or marble, which are both CaCOiii, were used as building materials, and gypsum (CaSO4·2 H2O) or lime (CaO) was used every bit mortar. Calcium sulfate is notwithstanding used in Portland cement and plaster of Paris. Magnesium and beryllium form lightweight, high-force alloys that are used in the aerospace, automotive, and other loftier-tech industries. As you learned in Chapter 6 "The Structure of Atoms", one of the near impressive uses of these elements is in fireworks; strontium and barium salts, for example, give red or green colors, respectively. Except for beryllium, which is highly toxic, the group ii elements are also important biologically. Bone is largely hydroxyapatite [Cav(PO4)3OH], mollusk shells are calcium carbonate, magnesium is office of the chlorophyll molecule in green plants, and calcium is of import in hormonal and nervus signal transmission. Considering BaSOiv is and so insoluble, information technology is used in "barium milk shakes" to obtain x-rays of the alimentary canal.
Group thirteen

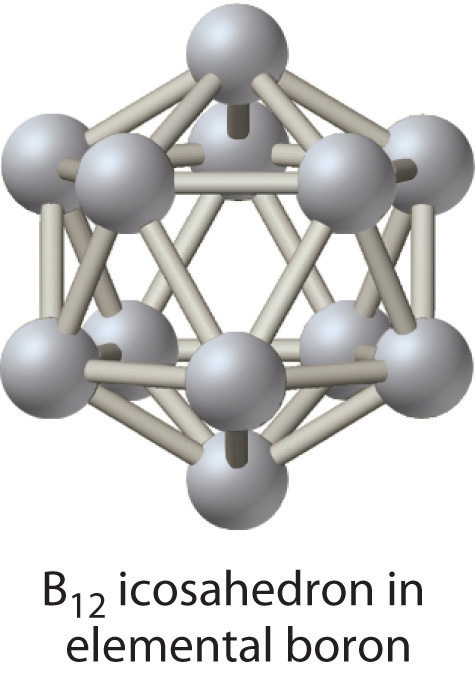
Of the group thirteen elements, just the lightest, boron, lies on the diagonal line that separates nonmetals and metals. Thus boron is a semimetal, whereas the balance of the group 13 elements are metals. Elemental boron has an unusual construction consisting of B12 icosahedra covalently bonded to i some other; the other elements are typical metallic solids.
No group 13 elements were known in ancient times, not because they are scarce—Al is the tertiary almost abundant element in Earth's crust—simply considering they are highly reactive and grade extremely stable compounds with oxygen. To isolate the pure elements, potent reducing agents and careful handling were needed.
The elements of grouping 13 have ns 2 np 1 valence electron configurations. Consequently, ii oxidation states are of import: +3, from losing 3 valence electrons to give the airtight-trounce electron configuration of the preceding noble gas; and +1, from losing the single electron in the np subshell. Considering these elements have small, negative electron affinities (boron'due south is only −27.0 kJ/mol), they are unlikely to larn five electrons to reach the side by side element of group 0 configuration. In fact, the chemical science of these elements is about exclusively characterized by +iii. Only the heaviest element (Tl) has extensive chemistry in the +1 oxidation land. It loses the single sixp electron to produce TlX monohalides and the oxide Tl2O.
In the 19th century, aluminum was considered a precious metal. In fact, information technology was considered and so precious that aluminum knives and forks were reserved for the French Emperor Louis Napoleon III, while his less of import guests had to be content with gilded or silver cutlery. Because of the metal's rarity the dedication of the Washington Monument in 1885 was celebrated by placing a 100 oz clamper of pure aluminum at the top. In contrast, today aluminum is used on an enormous scale in aircraft, automobile engines, armor, cookware, and beverage containers. It is valued for its combination of low density, high strength, and corrosion resistance. Aluminum is also constitute in compounds that are the active ingredients in about antiperspirant deodorants.
Compounds of boron, such as one form of BN, are hard, have a high melting point, and are resistant to corrosion. They are particularly useful in materials that are exposed to extreme weather condition, such as aircraft turbines, brake linings, and polishing compounds. Boron is also a major component of many kinds of glasses, and sodium perborate [NatwoB2O4(OH)4] is the active ingredient in many so-chosen color-safe laundry bleaches.
Gallium, indium, and thallium are less widely used, only gallium arsenide is the scarlet light-emitting diode (LED) in digital readouts in electronics, and MgGa2Oiv produces the greenish light emitted in many xerographic machines. Compounds of thallium(I) are extremely toxic. Although Tl2SO4 is an excellent rat or pismire poisonous substance, it is so toxic to humans that it is no longer used for this purpose.
Grouping xiv

The grouping 14 elements straddle the diagonal line that divides nonmetals from metals. Of the elements in this grouping, carbon is a nonmetal, silicon and germanium are semimetals, and tin and atomic number 82 are metals. As a result of this variety, the structures of the pure elements vary greatly.
The ns ii np ii valence electron configurations of group 14 gives rise to three oxidation states: −iv, in which iv electrons are added to attain the closed-shell electron configuration of the adjacent noble gas; +four, in which all four valence electrons are lost to give the closed-shell electron configuration of the preceding noble gas; and +ii, in which the loss of two np two electrons gives a filled ns 2 subshell.
The electronegativity of carbon is just 2.five, placing it in the middle of the electronegativity range, and then carbon forms covalent compounds with a wide multifariousness of elements and is the basis of all organic compounds. All of the group 14 elements form compounds in the +4 oxidation land, so all of them are able to course dioxides (from COtwo to PbO2) and tetrachlorides (CClfour and PbCl4). But the two metallic elements, Sn and Atomic number 82, form an all-encompassing series of compounds in the +2 oxidation country. Tin can salts are sprayed onto glass to brand an electrically conductive blanket, and then the drinking glass is used in the manufacture of frost-free windshields. Pb sulfate is formed when your auto battery discharges.
Carbon has at to the lowest degree 4 allotropes (forms or crystal structures) that are stable at room temperature: graphite; diamond; a group of related cage structures called fullerenesOne of at to the lowest degree four allotropes of carbon comprising a group of related cage structures. (such equally Cthreescore); and nanotubes1 of at to the lowest degree 4 allotropes of carbon that are cylinders of carbon atoms and are intermediate in construction betwixt graphite and the fullerenes. , which are cylinders of carbon atoms (Figure 7.eighteen "Four Allotropes of Carbon"). Graphite consists of extended planes of covalently bonded hexagonal rings. Considering the planes are not linked past covalent bonds, they tin slide beyond one some other easily. This makes graphite ideally suited as a lubricant and every bit the "lead" in pb pencils. Graphite also provides the blackness color in inks and tires, and graphite fibers are used in high-tech items such as golf game clubs, tennis rackets, airplanes, and sailboats because of their lightweight, forcefulness, and stiffness.
Figure 7.eighteen 4 Allotropes of Carbon
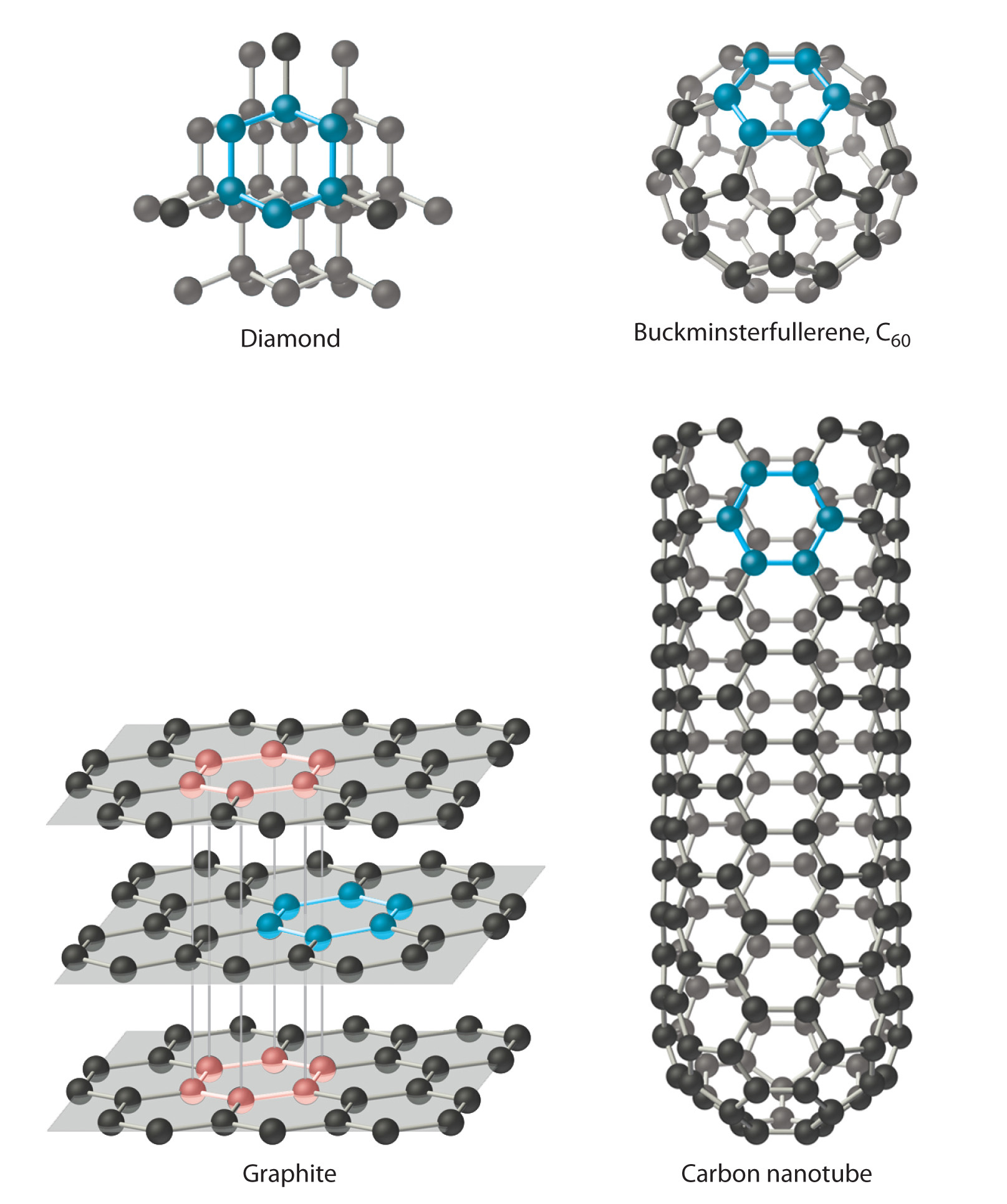
Diamond consists of a rigid 3-dimensional assortment of carbon atoms, making it one of the hardest substances known. In dissimilarity, graphite forms from extended planes of covalently bonded hexagonal rings of carbon atoms that can slide across one another hands. Fullerenes are spherical or ellipsoidal molecules with half dozen- and v-membered rings of carbon atoms, and nanotubes are sheets of graphite rolled upward into a cylinder.
In contrast to the layered structure of graphite, each carbon cantlet in diamond is bonded to four others to class a rigid three-dimensional array, making diamond 1 of the hardest substances known; consequently, information technology is used in industry as a cutting tool. Fullerenes, on the other hand, are spherical or oblong molecules with six- and five-membered rings of carbon atoms; they are volatile substances that dissolve in organic solvents. Fullerenes of extraterrestrial origin take been constitute in meteorites and have been discovered in a cloud of cosmic dust surrounding a distant star, which makes them the largest molecules ever seen in space. Carbon nanotubes, intermediate in structure betwixt graphite and the fullerenes, can be described as sheets of graphite that have been rolled up into a cylinder or, alternatively, fullerene cages that take been stretched in 1 management. Carbon nanotubes are being studied for use in the construction of molecular electronic devices and computers. For example, fabrics that are dipped in an ink of nanotubes and and then pressed to thin out the blanket are turned into batteries that maintain their flexibility. This creates "wearable electronics" and allows for the possibility of incorporating electronics into flexible surfaces. When applied to a t-shirt, for example, the t-shirt is converted into an "e-shirt."
Silicon is the 2d must abundant element in Globe's crust. Both silicon and germanium take strong, three-dimensional network structures similar to that of diamond. Sand is primarily SiO2, which is used commercially to brand glass and prevent caking in nutrient products. Complex compounds of silicon and oxygen with elements such as aluminum are used in detergents and talcum pulverization and every bit industrial catalysts. Because silicon-chip applied science laid the foundation for the modern electronics industry, the San Jose region of California, where many of the most important advances in electronics and computers were developed, has been nicknamed "Silicon Valley."
Elemental tin can and atomic number 82 are metal solids. Tin is primarily used to brand alloys such every bit bronze, which consists of tin and copper; solder, which is tin and lead; and pewter, which is tin can, antimony, and copper.
In aboriginal times, lead was used for everything from pipes to cooking pots because it is easily hammered into different shapes. In fact, the term plumbing is derived from plumbum, the Latin name for lead. Lead compounds were used as pigments in paints, and tetraethyllead was an important antiknock amanuensis in gasoline. Now, however, lead has been banned from many uses because of its toxicity, although it is all the same widely used in atomic number 82 storage batteries for automobiles. In previous centuries, lead salts were ofttimes used as medicines. Evidence suggests, for example, that Beethoven'due south decease was caused by the application of diverse lead-containing medicines by his dr.. Beethoven contracted pneumonia and was treated with lead salts, but in add-on, he suffered from a serious liver ailment. His physician treated the ailment by repeatedly puncturing his abdominal cavity and and then sealing the wound with a lead-laced poultice. It seems that the repeated doses of pb compounds contributed to Beethoven'southward death.
Group 15: The Pnicogens

The group 15 elements are called the pnicogensThe elements in grouping xv of the periodic table. —from the Greek pnigein, meaning "to choke," and genes, meaning "producing"—ostensibly because of the baneful fumes that many nitrogen and phosphorus compounds produce. This family has five stable elements; one isotope of bismuth (209Bi) is nonradioactive and is the heaviest nonradioactive isotope of any element. In one case again, the lightest fellow member of the family unit has unique properties. Although both nitrogen and phosphorus are nonmetals, nitrogen under standard conditions is a diatomic gas (N2), whereas phosphorus consists of three allotropes: white, a volatile, low-melting solid consisting of P4 tetrahedra; a red solid comprised of P8, P9, and Pten cages linked by P2 units; and black layers of corrugated phosphorus sheets. The side by side two elements, arsenic and antimony, are semimetals with extended three-dimensional network structures, and bismuth is a silvery metallic with a pinkish tint.
All of the pnicogens accept ns 2 np 3 valence electron configurations, leading to 3 common oxidation states: −three, in which three electrons are added to give the closed-shell electron configuration of the next noble gas; +5, in which all five valence electrons are lost to give the closed-shell electron configuration of the preceding noble gas; and +iii, in which only the three np electrons are lost to give a filled ns 2 subshell. Considering the electronegativity of nitrogen is similar to that of chlorine, nitrogen accepts electrons from about elements to form compounds in the −3 oxidation land (such as in NH3). Nitrogen has only positive oxidation states when combined with highly electronegative elements, such every bit oxygen and the halogens (east.m., HNO3, NF3). Although phosphorus and arsenic tin can combine with agile metals and hydrogen to produce compounds in which they have a −3 oxidation state (PH3, for case), they typically attain oxidation states of +3 and +five when combined with more electronegative elements, such as PCliii and H3PO4. Antimony and bismuth are relatively unreactive metals, merely form compounds with oxygen and the halogens in which their oxidation states are +3 and +5 (as in BitwoO3 and SbF5).
Although it is present in nearly biological molecules, nitrogen was the final pnicogen to be discovered. Nitrogen compounds such as ammonia, nitric acid, and their salts are used agriculturally in huge quantities; nitrates and nitrites are used as preservatives in meat products such every bit ham and bacon, and nitrogen is a component of nearly all explosives.
Phosphorus, too, is essential for life, and phosphate salts are used in fertilizers, toothpaste, and baking powder. I, phosphorus sulfide, P4South3, is used to ignite modernistic safety matches. Arsenic, in contrast, is toxic; its compounds are used equally pesticides and poisons. Antimony and bismuth are primarily used in metal alloys, just a bismuth compound is the active ingredient in the pop antacid medication Pepto-Bismol.
Group 16: The Chalcogens

The group xvi elements are often referred to equally the chalcogensThe elements in group 16 of the periodic tabular array. —from the Greek chalk, pregnant "copper," and genes, pregnant "producing"—because the nearly ancient copper ore, copper sulfide, is besides rich in two other group sixteen elements: selenium and tellurium. Once again, the lightest fellow member of the family unit has unique properties. In its most common pure class, oxygen is a diatomic gas (Oii), whereas sulfur is a volatile solid with Southward8 rings, selenium and tellurium are gray or silver solids that have bondage of atoms, and polonium is a silvery metal with a regular assortment of atoms. Like astatine and radon, polonium is a highly radioactive metallic element.
All of the chalcogens have ns 2 np 4 valence electron configurations. Their chemistry is dominated by iii oxidation states: −2, in which ii electrons are added to achieve the closed-beat out electron configuration of the next noble gas; +6, in which all 6 valence electrons are lost to give the closed-vanquish electron configuration of the preceding element of group 0; and +4, in which only the four np electrons are lost to give a filled ns 2 subshell. Oxygen has the 2d highest electronegativity of any element; its chemistry is dominated by the −2 oxidation state (every bit in MgO and HiiO). No compounds of oxygen in the +4 or +6 oxidation state are known. In dissimilarity, sulfur tin form compounds in all three oxidation states. Sulfur accepts electrons from less electronegative elements to give H2S and Na2S, for case, and information technology donates electrons to more electronegative elements to requite compounds such as SOii, Thenthree, and SF6. Selenium and tellurium, about the diagonal line in the periodic tabular array, behave similarly to sulfur merely are somewhat more than probable to be found in positive oxidation states.
Oxygen, the second virtually electronegative element in the periodic table, was not discovered until the late 18th century, even though it constitutes twenty% of the atmosphere and is the almost abundant element in Earth's crust. Oxygen is essential for life; our metabolism is based on the oxidation of organic compounds by O2 to produce CO2 and H2O. Commercially, oxygen is used in the conversion of pig iron to steel, equally the oxidant in oxyacetylene torches for cutting steel, as a fuel for the Us space shuttle, and in infirmary respirators.
Sulfur is the brimstone in "fire and brimstone" from ancient times. Partly as a effect of its long history, it is employed in a wide variety of commercial products and processes. In fact, as you learned in Affiliate 2 "Molecules, Ions, and Chemical Formulas", more sulfuric acid is produced worldwide than whatever other chemical compound. Sulfur is used to cross-link the polymers in rubber in a process chosen vulcanization, which was discovered by Charles Goodyear in the 1830s and commercialized by Benjamin Goodrich in the 1870s. Vulcanization gives rubber its unique combination of force, elasticity, and stability.
Selenium, the but other commercially of import chalcogen, was discovered in 1817, and today it is widely used in light-sensitive applications. For example, photocopying, or xerography, from the Greek xèrós, meaning "dry out," and graphia, meaning "writing," uses selenium films to transfer an image from i piece of paper to another, while compounds such as cadmium selenide are used to mensurate calorie-free in photographic calorie-free meters and automatic streetlights.
Group 17: The Halogens

The term halogen, derived from the Greek háls, meaning "salt," and genes, meaning "producing," was first applied to chlorine because of its tendency to react with metals to form salts. All of the halogens have an ns 2 np 5 valence electron configuration, and all merely astatine are diatomic molecules in which the two element of group vii atoms share a pair of electrons. Diatomic Ftwo and Cl2 are pale xanthous-green and pale green gases, respectively, while Brii is a red liquid, and Iii is a purple solid. The halogens were non isolated until the 18th and 19th centuries.
Because of their relatively high electronegativities, the halogens are nonmetallic and generally react by gaining one electron per cantlet to achieve a noble gas electron configuration and an oxidation state of −1. Halides are produced according to the following equation, in which X denotes a element of group vii:
Equation 7.16
2 E +n10ii → 2 EX north
If the element E has a low electronegativity (as does Na), the product is typically an ionic halide (NaCl). If the element E is highly electronegative (equally P is), the product is typically a covalent halide (PClfive). Ionic halides tend to exist nonvolatile substances with high melting points, whereas covalent halides tend to be volatile substances with depression melting points. Fluorine is the about reactive of the halogens, and iodine the to the lowest degree, which is consistent with their relative electronegativities (Figure vii.xv "Pauling Electronegativity Values of the ").Every bit we shall see in subsequent capacity, however, factors such as bail strengths are besides of import in dictating the reactivities of these elements. In fact, fluorine reacts with nearly all elements at room temperature. Under more extreme conditions, it combines with all elements except helium, neon, and argon.
The halogens react with hydrogen to class the hydrogen halides (HX):
Equation 7.17
Htwo(g) + Xii(g,fifty,s) → 2 HX(g)
Fluorine is so reactive that whatever substance containing hydrogen, including coal, woods, and fifty-fifty water, will outburst into flames if it comes into contact with pure F2.
Because it is the most electronegative element known, fluorine never has a positive oxidation state in any compound. In contrast, the other halogens (Cl, Br, I) grade compounds in which their oxidation states are +1, +3, +5, and +7, as in the oxoanions, XO n −, where north = ane–4. Because oxygen has the second highest electronegativity of whatsoever element, it stabilizes the positive oxidation states of the halogens in these ions.
All of the halogens except astatine (which is radioactive) are commercially of import. NaCl in table salt water is purified for use as table salt. Chlorine and hypochlorite (OCl−) salts are used to sanitize public water supplies, swimming pools, and wastewater, and hypochlorite salts are likewise used as bleaches because they oxidize colored organic molecules. Organochlorine compounds are used as drugs and pesticides. Fluoride (commonly in the grade of NaF) is added to many municipal water supplies to help forbid tooth disuse, and bromine (in AgBr) is a component of the light-sensitive coating on photographic film. Because iodine is essential to life—it is a central component of the hormone produced by the thyroid gland—small amounts of KI are added to common salt to produce "iodized common salt," which prevents thyroid hormone deficiencies.
Grouping 18: The Noble Gases
The noble gases are helium, neon, argon, krypton, xenon, and radon. All have filled valence electron configurations and therefore are unreactive elements constitute in nature every bit monatomic gases. The noble gases were long referred to as either "rare gases" or "inert gases," just they are neither rare nor inert. Argon constitutes nearly 1% of the temper, which also contains small amounts of the lighter group 18 elements, and helium is plant in large amounts in many natural gas deposits. The grouping's perceived "rarity" stems in part from the fact that the noble gases were the last major family of elements to exist discovered.
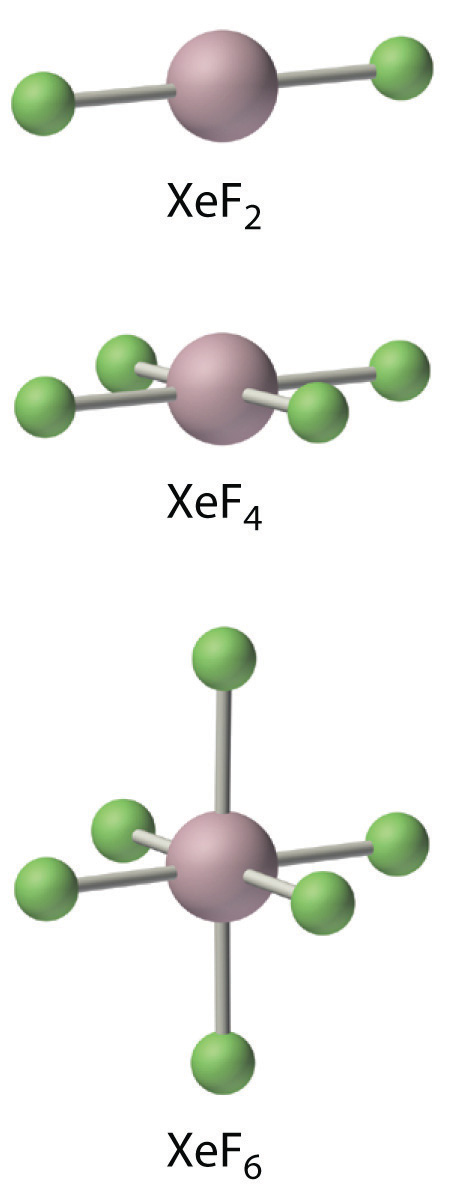
The noble gases take EA ≥ 0, so they do not form compounds in which they have negative oxidation states. Because ionization energies decrease down the column, the only noble gases that grade compounds in which they accept positive oxidation states are Kr, Xe, and Rn. Of these iii elements, only xenon forms an extensive series of compounds. The chemistry of radon is severely limited by its extreme radioactive decay, and the chemical science of krypton is limited by its high ionization energy (1350.8 kJ/mol versus 1170.4 kJ/mol for xenon). In essentially all its compounds, xenon is bonded to highly electronegative atoms such as fluorine or oxygen. In fact, the only significant reaction of xenon is with elemental fluorine, which can requite XeFii, XeF4, or XeF6. Oxides such equally XeO3 are produced when xenon fluorides react with water, and oxidation with ozone produces the perxenate ion [XeOhalf dozen 4−], in which xenon acquires a +8 oxidation land past formally donating all eight of its valence electrons to the more electronegative oxygen atoms. In all of its stable compounds, xenon has a positive, even-numbered oxidation state: +2, +iv, +6, or +8. The actual stability of these compounds varies greatly. For example, XeO3 is a daze-sensitive, white crystalline solid with explosive ability comparable to that of TNT (trinitrotoluene), whereas another compound, NaiiXeF8, is stable up to 300°C.
Although none of the noble gas compounds is commercially significant, the elements themselves take important applications. For example, argon is used in incandescent calorie-free bulbs, where information technology provides an inert atmosphere that protects the tungsten filament from oxidation, and in compact fluorescent light bulbs (CFLs). Information technology is also used in arc welding and in the manufacture of reactive elements, such as titanium, or of ultrapure products, such as the silicon used by the electronics industry. Helium, with a boiling signal of only 4.2 K, is used as a liquid for studying the properties of substances at very low temperatures. It is too combined in an 80:20 mixture with oxygen used by scuba divers, rather than compressed air, when they descend to corking depths. Because helium is less soluble in h2o than Nii—a component of compressed air—replacing N2 with He prevents the germination of bubbles in claret vessels, a condition called "the bends" that can occur during rapid ascents. Neon is familiar to all of the states as the gas responsible for the red glow in neon lights.
The Transition Metals, the Lanthanides, and the Actinides
As expected for elements with the same valence electron configuration, the elements in each column of the d cake have vertical similarities in chemical behavior. In contrast to the southward- and p-cake elements, however, elements in the d block also brandish potent horizontal similarities. The horizontal trends compete with the vertical trends. In further contrast to the p-block elements, which tend to have stable oxidation states that are separated by two electrons, the transition metalsAny element in groups 3–12 in the periodic table. All of the transition elements are metals. have multiple oxidation states that are separated by simply one electron.
Note the Blueprint
The p-block elements form stable compounds in oxidation states that tend to be separated by ii electrons, whereas the transition metals have multiple oxidation states that are separated past one electron.
The group 6 elements, chromium, molybdenum, and tungsten, illustrate the competition that occurs between these horizontal and vertical trends. For case, the maximum oxidation state for all elements in group 6 is +6, achieved by losing all six valence electrons (think that Cr has a 4s 1threed five valence electron configuration), yet near all the elements in the showtime row of the transition metals, including chromium, form compounds with the dication M2+, and many also class the trication Miii+. Equally a upshot, the transition metals in group 6 have very dissimilar tendencies to achieve their maximum oxidation state. The near common oxidation state for chromium is +iii, whereas the nigh common oxidation country for molybdenum and tungsten is +half dozen.
Note the Pattern
The d-cake elements display both strong vertical and horizontal similarities.
Groups 3 (scandium, lanthanum, actinium), 11 (copper, silver, gold), and 12 (zinc, cadmium, mercury) are the only transition metal groups in which the oxidation land predicted by the valence electron configuration dominates the chemistry of the group. The elements of group 3 accept iii valence electrons exterior an inner airtight vanquish, so their chemistry is almost exclusively that of the M3+ ions produced past losing all three valence electrons. The elements of group 11 have 11 valence electrons in an ns 1(n − 1)d 10 valence electron configuration, and and so all iii lose a single electron to form the monocation G+ with a closed (n − 1)d x electron configuration. Consequently, compounds of Cu+, Ag+, and Au+ are very common, although in that location is also a great deal of chemical science involving Cu2+. Similarly, the elements of group 12 all have an ns 2(n − 1)d 10 valence electron configuration, so they lose two electrons to form Yard2+ ions with an (n − i)d x electron configuration; indeed, the well-nigh important ions for these elements are Zn2+, Cdtwo+, and Hgii+. Mercury, however, likewise forms the dimeric mercurous ion (Hg2 2+) because of a subtle residuum betwixt the energies needed to remove additional electrons and the energy released when bonds are formed. The +three oxidation state is the most of import for the lanthanidesAny of the 14 elements betwixt (cerium) and (lutetium). and for about of the actinidesAny of the 14 elements between (thorium) and (lawrencium). .
Case viii
Based on the post-obit information, determine the most likely identities for elements D and East.
- Chemical element D is a shiny gray solid that conducts electricity only moderately; it forms two oxides (Practiceii and Do3).
- Element Eastward is a reddish metal substance that is an excellent conductor of electricity; it forms two oxides (EO and EasttwoO) and two chlorides (ECl and ECl2).
Given: physical and chemical properties of two elements
Asked for: identities
Strategy:
A Based on the conductivity of the elements, determine whether each is a metal, a nonmetal, or a semimetal. Ostend your prediction from its concrete appearance.
B From the compounds each element forms, determine its common oxidation states.
C If the element is a nonmetal, it must exist located in the p cake of the periodic table. If a semimetal, information technology must lie along the diagonal line of semimetals from B to At. Transition metals can take two oxidation states separated by one electron.
D From your nomenclature, the oxidation states of the element, and its physical appearance, deduce its identity.
Solution:
- A The moderate conductivity of element D tells us that it is a semimetal. It must lie in the p block of the periodic table because all of the semimetals are located there. B The stoichiometry of the oxides tells us that two common oxidation states for D are +4 and +6. C Chemical element D must be located in grouping 16 considering the mutual oxidation states for the chalcogens (group xvi) include +6 (past losing all half dozen valence elections) and +4 (past losing the four electrons from the p subshell). Thus D is likely to be Se or Te. D Additional data is needed to distinguish between the two.
- A Element Eastward is an splendid electrical conductor, so information technology is a metal. B The stoichiometry of the oxides and chlorides, nevertheless, tells us that common oxidation states for Due east are +ii and +1. C Metals that tin have two oxidation states separated by ane electron are normally transition metals. The +1 oxidation land is characteristic of just ane group: grouping 11. Inside group 11, copper is the simply element with common oxidation states of +1 and +2. D Copper also has a reddish hue. Thus element E is probably copper.
Exercise
Based on the following information, determine the most likely identities for elements G and J.
- Element G is a scarlet liquid that does non bear electricity. It forms three compounds with fluorine (GF, GF3, and GFv) and one with sodium (NaG).
- Element J is a soft, deadening gray solid that conducts electricity well and forms 2 oxides (JO and JO2).
Respond:
- Br
- Sn or Lead
Summary
The chemical families consist of elements that have the aforementioned valence electron configuration and tend to accept similar chemistry. The alkali metals (group 1) have ns one valence electron configurations and form M+ ions, while the alkaline earth metals (group 2) have ns 2 valence electron configurations and form Kii+ ions. Group 13 elements take ns two np 1 valence electron configurations and have an overwhelming tendency to course compounds in the +3 oxidation state. Elements in group 14 have ns ii np 2 valence electron configurations but showroom a diversity of chemical behaviors because they range from a nonmetal (carbon) to metals (tin can/pb). Carbon, the basis of organic compounds, has at to the lowest degree four allotropes with singled-out structures: diamond, graphite, fullerenes, and carbon nanotubes. The pnicogens (grouping 15) all take ns 2 np 3 valence electron configurations; they form compounds in oxidation states ranging from −3 to +5. The chalcogens (grouping 16) have ns ii np 4 valence electron configurations and react chemically by either gaining 2 electrons or past formally losing four or six electrons. The halogens (group 17) all have ns 2 np 5 valence electron configurations and are diatomic molecules that tend to react chemically by accepting a single electron. The noble gases (group 18) are monatomic gases that are chemically quite unreactive due to the presence of a filled shell of electrons. The transition metals (groups 3–10) contain partially filled sets of d orbitals, and the lanthanides and the actinides are those groups in which f orbitals are being filled. These groups exhibit strong horizontal similarities in behavior. Many of the transition metals form M2+ ions, whereas the chemistry of the lanthanides and actinides is dominated by M3+ ions.
Fundamental Takeaway
- Periodic backdrop and the chemical behavior of the elements correlate strongly with valence electron configurations and Mulliken electronegativities.
Conceptual Bug
-
Of the grouping i elements, which would you await to be the best reductant? Why? Would you await boron to be a skillful reductant? Why or why not?
-
Classify each element as a metal, a nonmetal, or a semimetal: Hf, I, Tl, S, Si, He, Ti, Li, and Sb. Which would you expect to exist proficient electrical conductors? Why?
-
Classify each element as a metal, a nonmetal, or a semimetal: Au, Bi, P, Kr, Five, Na, and Po. Which would you expect to be skilful electrical insulators? Why?
-
Of the elements Kr, Xe, and Ar, why does but xenon class an extensive series of compounds? Would you lot expect Xe2+ to be a good oxidant? Why or why non?
-
Identify each statement about the halogens every bit either truthful or false and explain your reasoning.
- Halogens have filled valence electron configurations.
- Halogens tend to form salts with metals.
- As the gratis elements, halogens are monatomic.
- Halogens have observable nonmetallic character.
- Halogens tend to take an oxidation state of −1.
- Halogens are skilful reductants.
-
Nitrogen forms compounds in the +five, +4, +iii, +ii, and −three oxidation states, whereas Bi forms ions only in the +five and +iii oxidation states. Propose an explanation for the differences in beliefs.
-
Of the elements Mg, Al, O, P, and Ne, which would you expect to form covalent halides? Why? How do the melting points of covalent halides compare with those of ionic halides?
-
Of the elements Li, Ga, Equally, and Xe, would you expect to form ionic chlorides? Explain your reasoning. Which are unremarkably more volatile—ionic or covalent halides? Why?
-
Predict the human relationship between the oxidative strength of the oxoanions of bromine—BrO due north − (north = i–four)—and the number of oxygen atoms nowadays (northward). Explicate your reasoning.
-
The stability of the binary hydrides of the chalcogens decreases in the order H2O > H2S > H2Se > HtwoTe. Why?
-
Of the elements O, Al, H, and Cl, which will form a compound with nitrogen in a positive oxidation state? Write a reasonable chemical formula for an example of a binary compound with each element.
-
How exercise you explain the differences in chemistry observed for the group fourteen elements as you become down the column? Classify each group xiv element every bit a metal, a nonmetal, or a semimetal. Practise yous await the group 14 elements to form covalent or ionic compounds? Explain your reasoning.
-
Why is the chemistry of the grouping 13 elements less varied than the chemistry of the group fifteen elements? Would you lot expect the chemistry of the group 13 elements to be more or less varied than that of the group 17 elements? Explain your reasoning.
-
If you lot needed to design a substitute for BaSO4, the barium milkshake used to examine the large and small intestine by x-rays, would BeSOiv be an inappropriate substitute? Explain your reasoning.
-
The alkali metals have an ns ane valence electron configuration, and consequently they tend to lose an electron to class ions with +1 charge. Based on their valence electron configuration, what other kind of ion tin can the alkali metals course? Explain your reply.
-
Would Mo or Due west be the more appropriate biological substitute for Cr? Explain your reasoning.
Answer
-
Nitrogen will have a positive oxidation state in its compounds with O and Cl, because both O and Cl are more electronegative than N. Reasonable formulas for binary compounds are: North2Ov or N2Oiii and NCl3.
Numerical Issues
-
Write a balanced equation for formation of XeO3 from elemental Xe and O2. What is the oxidation state of Xe in XeO3? Would you expect Ar to undergo an coordinating reaction? Why or why not?
-
Which of the p-block elements exhibit the greatest variation in oxidation states? Why? Based on their valence electron configurations, identify these oxidation states.
-
Based on its valence electron configuration, what are the three common oxidation states of selenium? In a binary compound, what atoms bonded to Se will stabilize the highest oxidation land? the lowest oxidation state?
-
Would you expect sulfur to be readily oxidized by HCl? Why or why not? Would yous expect phosphorus to be readily oxidized past sulfur? Why or why not?
-
What are the most common oxidation states for the pnicogens? What factors determine the relative stabilities of these oxidation states for the lighter and the heavier pnicogens? What is likely to be the most common oxidation state for phosphorus and arsenic? Why?
-
Of the compounds NFiii, NCl3, and NI3, which would exist the least stable? Explain your answer. Of the ions BrO−, ClO−, or FO−, which would be the least stable? Explain your answer.
-
In an attempt to explore the chemistry of the superheavy element ununquadium, Z = 114, you isolated 2 singled-out salts past exhaustively oxidizing metal samples with chlorine gas. These salts are found to have the formulas MCl2 and MCl4. What would be the name of ununquadium using Mendeleev's eka-notation?
-
Would you expect the compound CCl2 to be stable? SnCl2? Why or why not?
-
A newly discovered element (Z) is a skillful conductor of electricity and reacts only slowly with oxygen. Reaction of 1 g of Z with oxygen under three different sets of weather condition gives products with masses of 1.333 chiliad, 1.668 g, and 1.501 g, respectively. To what family of elements does Z belong? What is the atomic mass of the element?
-
An unknown element (Z) is a tedious, brittle powder that reacts with oxygen at high temperatures. Reaction of 0.665 gram of Z with oxygen under two different sets of weather forms gaseous products with masses of ane.328 thou and i.660 1000. To which family of elements does Z belong? What is the atomic mass of the element?
-
Why are the brine metals such powerful reductants? Would you expect Li to exist able to reduce H2? Would Li reduce Five? Why or why not?
-
What practice you predict to exist the most common oxidation state for Au, Sc, Ag, and Zn? Give the valence electron configuration for each chemical element in its nigh stable oxidation country.
-
Complete the following table.
Mg C Ne Fe Br Valence Electron Configuration Common Oxidation States Oxidizing Strength -
Use the following data to identify elements T, 10, D, and Z. Element T reacts with oxygen to form at to the lowest degree three compounds: TO, T2O3, and TO2. Element X reacts with oxygen to grade XO2, just Ten is also known to form compounds in the +ii oxidation state. Element D forms DiiO3, and chemical element Z reacts vigorously and forms Z2O. Electric conductivity measurements showed that element X exhibited electrical conductivity intermediate between metals and insulators, while elements T, D, and Z were good conductors of electricity. Element T is a hard, lustrous, silvery metal, element X is a blue-gray metal, element D is a light, silver metallic, and element Z is a soft, depression-melting metal.
-
Predict whether Cs, Fii, Al, and He will react with oxygen. If a reaction will occur, identify the products.
-
Predict whether One thousand, Ar, O, and Al will react with Cl2. If a reaction volition occur, identify the products.
-
Use the post-obit information to identify elements X, T, and Z.
- Element 10 is a soft, silverish-white metal that is flammable in air and reacts vigorously with water. Its outset ionization energy is less than 500 kJ/mol, but the 2nd ionization energy is greater than 3000 kJ/mol.
- Element T is a gas that reacts with F2 to form a series of fluorides ranging from TF2 to TF6. It is inert to most other chemicals.
- Element Z is a deep red liquid that reacts with fluorine to form ZFthree and with chlorine to course ZCl and ZClthree, and with iodine to form ZI. Element Z too reacts with the alkali metals and alkaline globe metals.
-
Calculation a reactive metallic to water in the presence of oxygen results in a burn. In the absence of oxygen, the addition of 551 mg of the metal to water produces 6.4 mg of hydrogen gas. Treatment of 2.00 m of this metal with six.iii g of Br2 results in the formation of 3.86 thousand of an ionic solid. To which chemical family unit does this element belong? What is the identity of the element? Write and balance the chemical equation for the reaction of h2o with the metallic to form hydrogen gas.
Answers
-
two Xe + three Otwo → 2 XeO3
The oxidation state of xenon in XeOthree is +6. No, Ar is much more than hard to oxidize than Xe.
-
The valence electron configuration of Se is [Ar]4s 23d 104p four. Its common oxidation states are: +6, due to loss of all six electrons in the 4southward and 4p subshells; +iv, due to loss of only the four 4p electrons; and −2, due to add-on of ii electrons to give an [Ar]4southward 23d 104p vi electron configuration, which is isoelectronic with the following element of group 0, Kr. The highest oxidation state (+six) will be stabilized by bonds to highly electronegative atoms such every bit F (SeF6) and O (SeO3), while the lowest oxidation state volition be stabilized in covalent compounds by bonds to less electronegative atoms such every bit H (HtwoSe) or C [(CH3)2Se], or in ionic compounds with cations of electropositive metals (NaiiSe).
-
All of the pnicogens accept ns 2 np 3 valence electron configurations. The pnicogens therefore tend to course compounds in three oxidation states: +5, due to loss of all five valence electrons; +three, due to loss of the three np three electrons; and −three, due to addition of three electrons to give a closed beat out electron configuration. Bonds to highly electronegative atoms such as F and O volition stabilize the college oxidation states, while bonds to less electronegative atoms such as H and C volition stabilize the lowest oxidation state, as will formation of an ionic compound with the cations of electropositive metals. The most common oxidation state for phosphorus and arsenic is +5.
-
Uuq =eka-atomic number 82
-
The ratios of the masses of the element to the mass of oxygen give empirical formulas of ZO, Z2O3, and ZO2. The high electrical conductivity of the chemical element immediately identifies it every bit a metallic, and the being of three oxides of the element with oxidation states separated past only 1 electron identifies information technology equally a transition metallic. If 1 one thousand of Z reacts with 0.33 thousand Oii to requite ZO, the balanced equation for the reaction must be 2 Z + O2 → ii ZO. Using M to represent tooth mass, the ratio of the molar masses of ZO and Z is therefore:
ThouZO:MZ = (MZ + ChiliadO): GZ = (MZ + 16.0): 1000Z = 1.33:ane = 1.33.
Solving for One thousandZ gives a molar mass of 48 g/mol and an atomic mass of 48 amu for Z, which identifies it equally titanium.
-
Alkali metals are powerful reductants because they have a strong tendency to lose their ns one valence electron, equally reflected in their depression first ionization energies and electronegativities. Lithium has a more positive electron affinity than hydrogen and a substantially lower get-go ionization free energy, and so nosotros await lithium to reduce hydrogen. Transition metals accept low electron affinities and do not normally form compounds in negative oxidation states. Therefore, nosotros do not await lithium to reduce vanadium.
-
Mg C Ne Fe Br Valence Electron Configuration 3s ii twos 22p 2 2s ii2p vi foursouthward 2iiid vi 4s ii4p v Mutual Oxidation States +two −4, +4 0 +2, +3 −1, +1, +3, +5, +vii Oxidizing Strength None Weak None None Strong -
4 Cs(s) + O2(one thousand) → two CstwoO(south) two F2(one thousand) + O2(1000) → OF2(g) 4 Al(s) + 3 O2(g) → 2 Al2Othree(s) He + O2(yard) → no reaction
-
- sodium or potassium
- xenon
- bromine
donaldsonpribue1956.blogspot.com
Source: https://saylordotorg.github.io/text_general-chemistry-principles-patterns-and-applications-v1.0/s11-04-the-chemical-families.html
0 Response to "What Other Properties Could Mendeleev Examine to Determine Families of Elements?"
Post a Comment